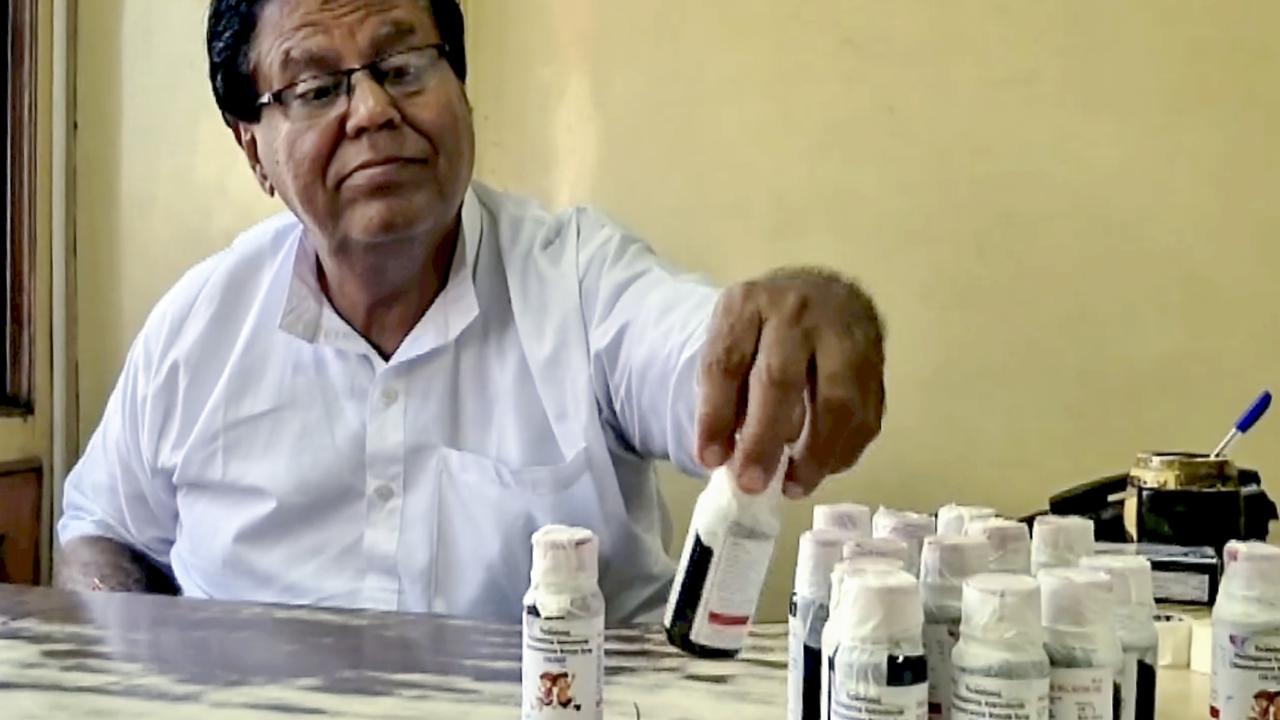
Consumer rights organisation Mumbai Grahak Panchayat on Friday urged the Central Consumer Protection Authority (CCPA) to recall Coldrif and other such cough syrups nationwide that are hazardous and unsafe, reported the PTI.
In a letter written to CCPA Chief Commissioner Nidhi Khare, the Mumbai Grahak Panchayat (MGP) has sought urgent action in response to the recent deaths of children in Madhya Pradesh and Rajasthan due to Diethylene Glycol (DEG)-contaminated cough syrup, according to the PTI.
The cough syrup, marketed as `Coldrif` and reportedly manufactured by Sresan Pharmaceuticals, has been banned in several states following laboratory confirmation of DEG contamination, a toxic industrial solvent known to cause kidney failure and death when ingested, the MGP said in the letter.
The MGP called for an immediate nationwide freeze and recall of Coldrif and other suspect syrups under Section 20 (a) of Consumer Protection Act 2019, claiming such hazardous and unsafe products seriously threaten consumers` right to safety.
The consumer rights organisation also urged for a complete ban on the use of DEG in paediatric formulations and stricter pharmacovigilance protocols.
It also asked CCPA to file a complaint with National Consumer Disputes Redressal Commission (NCDRC) seeking compensation for kin of the deceased children.
MGP said this is not an isolated incident as there have been such DEG-linked fatalities in Jammu (2020), Gurgaon (1998), and Chennai (1972), as well as international cases involving Indian exports to The Gambia and Uzbekistan, the news agency reported on Friday.
“This tragedy is a stark violation of the Right to Safety enshrined in the Consumer Protection Act. We urge the CCPA to act decisively and restore public trust in drug safety,” MGP chairperson Shirish V Deshpande asserted, the PTI reported.
Meanwhile, Tamil Nadu’s Health Minister, Ma Subramanian on Friday said that the state government had warned the Madhya Pradesh government, the Union Health Ministry, and other states about the presence of a toxic substance in Coldrif cough syrup, potentially averting a major public health crisis.
The minister’s comments come amid criticism from AIADMK general secretary Edappadi K. Palaniswami and the Madhya Pradesh government, following the reported deaths of children allegedly linked to the adulterated cough syrup, which was manufactured in Tamil Nadu, as per the PTI.
Subramanian rejected allegations of regulatory failure, asserting that the state took swift and responsible action. He said that on 1 October, the Principal Secretary of the Health Department ordered a probe into the adulteration. Tests later revealed that Coldrif syrup contained 48% Diethylene Glycol, a highly toxic industrial chemical.
“We acted immediately by alerting the Madhya Pradesh government and the Union Health Ministry, as well as notifying authorities in Odisha and Puducherry,” Subramanian told reporters.
“However, at the time, they responded saying the medicine was fine,” he said.
The Tamil Nadu government maintains that its timely intervention helped prevent further tragedy, even as questions continue to be raised over the source of the contamination and regulatory oversight.
(with PTI inputs)